
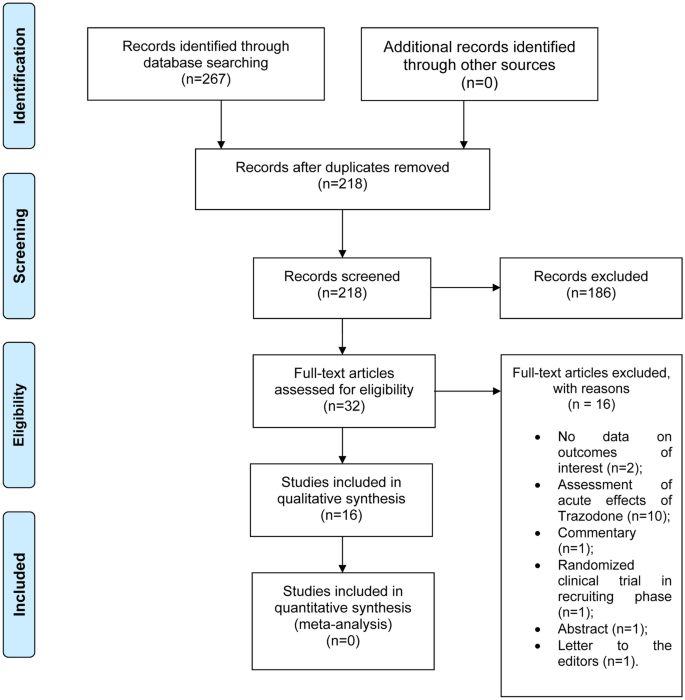
Trazodone is a widely used antidepressant that is also useful in the control of agitation and insomnia in Alzheimer's disease. This drug is now recognized as having a new mechanism of action, an effect on the unfolded protein response (UPR) pathway, restoring protein translation and slowing neurodegenerative progression in mice. This mechanism may have a role in dementia-modifying treatment. To explore the effects of trazodone on human cognition and to search for clinical evidence of its putative benefits in human neurodegenerative diseases, a systematic review was conducted for studies that evaluated the effect of a minimum dose of 25 mg of trazodone daily, for at least 1 week, on cognition in adult humans. The search was run in MEDLINE, Web of Science, and CENTRAL from the Cochrane databases, yielding a total of 16 studies after selection. Overall, seven studies showed no effect of trazodone on cognition, five showed a beneficial effect by improving or reducing cognitive decline, and four evidenced impaired cognitive function. Our analysis highlights the possibility of a dose-independent dual effect of trazodone on human cognition, with acute utilization associated with impaired cognitive function and long-term use with preventing cognitive deterioration. There was no clinical evidence that trazodone could be used as a specific treatment of neurodegenerative diseases. Future studies should explore the role of trazodone in the UPR pathway and the implications in neurodegenerative diseases in humans.

Avoid common mistakes on your manuscript.
Although it is FDA-approved only for use in the treatment of major depression, trazodone, a drug created in the 1960s [1], is widely used off-label to control agitation and insomnia in Alzheimer’s disease (AD) [2] and in anxiety, schizophrenia, bulimia, substance abuse, fibromyalgia [3], and post-traumatic stress disorder [1, 4]. Additionally, it reduces behavioural and psychological symptoms in AD and frontotemporal dementia [2].
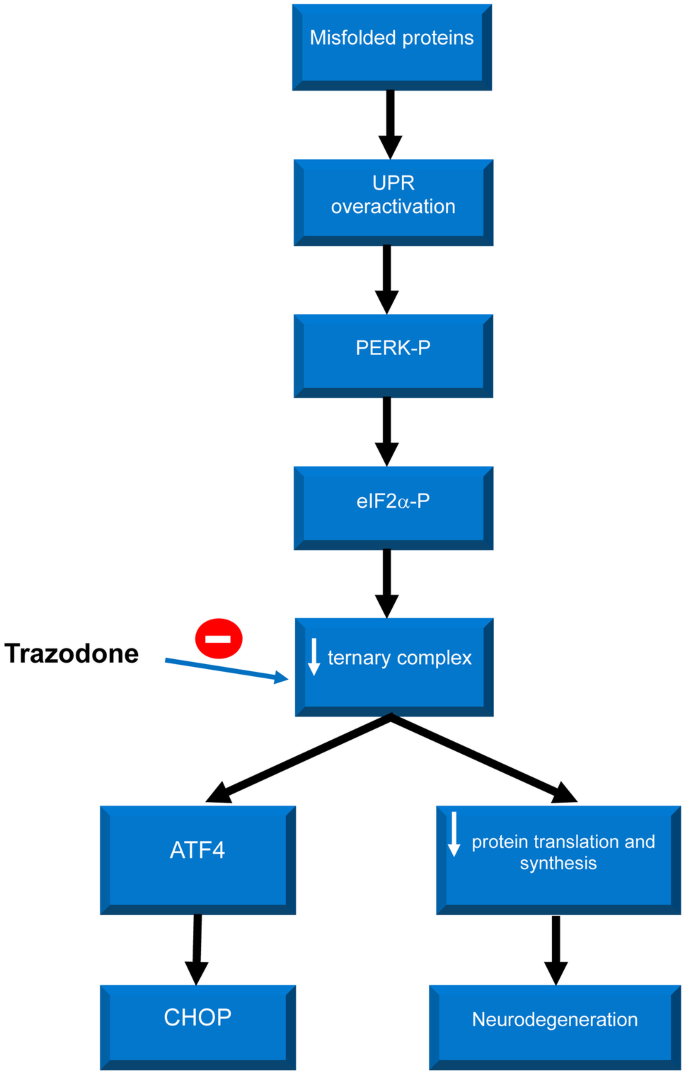
Trazodone is now recognized as having a novel mechanism of action, its effect in the unfolded protein response (UPR) pathway. Trazodone acts downstream of eIF2α-P, preventing it from reducing levels of the ternary complex and allowing protein translation to occur [2] (see Supplementary Fig. 1). Hence, trazodone has been shown to prevent the effects of UPR overactivation observed in neurodegenerative diseases [2] and restore neuronal protein synthesis, preventing neurodegeneration in mouse models [5]. As a result, this drug appears to have utility as a novel disease-modifying treatment for neurodegenerative diseases in humans [2].
However, if trazodone has a role in preventing cognitive decline, it is important to consider whether that effect is mediated through its action in the UPR pathway, by increasing the synaptic concentration of 5-hydroxytryptamine [6], or as a result of the sleep improvement this drug offers. Establishing the effect of trazodone on cognition and neurodegeneration would have significant implications for clinical practice, given the increasing prevalence of neurodegenerative diseases and the extensive use of trazodone in this patient population.
Thus, we aimed to establish the effects of trazodone on human cognition and to determine whether there was evidence that it could be used in the treatment of neurodegenerative diseases in humans.
Following PRISMA [Preferred Reporting Items for Systematic Reviews and Meta-Analyses] guidelines, we conducted a systematic review, without a meta-analysis, of studies that evaluated the effect of trazodone on human cognition.
Our target population was healthy or diseased adults aged 18 years or older. The diseased patients could have all types of illness, from atherosclerotic disease to psychiatric conditions. We excluded animal studies, since our focus was on the consequences in humans in order to assess the implications for clinical practice.
The targeted intervention was a minimum dose of 25 mg of trazodone daily, for at least 1 week, to observe the chronic effects of this drug and not the effects obtained after a single dose. Consequently, we excluded studies that only tested the acute effects of trazodone by using it in a one-time-only fashion. The dose of 25 mg was chosen because it is the minimum dose used that is capable of producing some effects of the drug observed in clinical practice. In terms of comparators, all comparators were accepted without restriction.
Our main outcome was to study the effect of trazodone on human cognition. To this end, we analysed all instruments that measured the cognitive impairment that appeared in the studies included in the qualitative synthesis. These instruments included the Montreal Cognitive Assessment scale (MoCA), Mini-Mental State Examination (MMSE), the Digit Span subtest, the d2 test, the Wisconsin Card Sorting Test, Continuous Performance Test, n-back Test, Paired Associate Learning Test-Form I (short-term memory), Paired Associate Learning Test-Form II (long-term memory) of the Wechsler Memory Scale, Arithmetic, Letter-Number Sequencing, Digit Symbol-Coding, Symbol Search of the Wechsler Adult Intelligence scale (third edition, WAIS-III), Buschke Selective Reminding Test, the Brown-Peterson Memory Test, the Word Learning Test, the Memory Scanning Test, the Critical Flicker/Fusion (CFF) frequency, the Critical Tracking Task, the Divided Attention Test, the Visual Vigilance Test, Rey's Verbal Memory (RVM) test, the Guild Memory Test, Trail Making Test, free recall test, Corsi block test, category generation, “News” recall, Who’s who? or matching to sample. Our secondary outcome was whether trazodone could be included in the treatment of neurodegenerative diseases in humans. To assess effects on neurodegeneration, we searched for reports of beneficial effects of trazodone in cognitive decline and studies including analyses of mechanisms through which that benefit could occur.
The studies included randomized controlled trials, non-randomized trials, and retrospective and prospective cohort studies. No limits in language or publication year were applied.
The literature search was done in electronic databases from September 14 to September 22, 2020. The search was conducted in MEDLINE (1990–present), Web of Science (1999–present), and CENTRAL from Cochrane (1994–present). The last search was run on November 2, 2020.
The following search terms were used to search in PubMed: trazodone; memory; memories; cognition; cognitions; cognitive; cognitively. The detailed search strategy can be assessed in supplementary Table 1.
Studies were selected in two phases independently by two reviewers. In the first phase, articles were chosen by their title and abstract. In the second phase, the articles selected in the previous phase were read in full to determine eligibility for inclusion. Data were collected manually and independently by the two reviewers and synthesized in tables. Disagreements between reviewers were resolved by discussion and consensus. For each study included, the following information was collected: the study author(s), title, year of publication and design, the follow-up period, the study size, the population being studied, its age and sex, the intervention under study, the comparators used, and the outcome measures (Tables 1 and 2). The effects of trazodone on human cognition were synthesized using a table to represent all scores of the cognitive evaluation scales obtained in order to determine the final result of the effect. The final result was divided into three categories: no effect (defined as neither improving nor impairing cognition), positive effect (defined as cognition improvement or delayed cognitive decline), and negative effect (defined as cognition impairment).
All data collected and analyzed are available in this article.
The authors declare no funding for this study.
Ana Mafalda Gonçalves Gonçalo contributed to the design and conceptualization of the study, and had a major role in the acquisition, analysis, and interpretation of the data. Maria Augusta Vieira-Coelho, MD, Ph.D. contributed to the design and conceptualization of the study, had a major role in the acquisition, analysis, and interpretation of the data, and revised the manuscript for intellectual content.
The authors declare no conflict of interest.
Below is the link to the electronic supplementary material.
Gonçalo, A.M.G., Vieira-Coelho, M.A. The effects of trazodone on human cognition: a systematic review. Eur J Clin Pharmacol 77, 1623–1637 (2021). https://doi.org/10.1007/s00228-021-03161-6
Anyone you share the following link with will be able to read this content:
Get shareable link
Sorry, a shareable link is not currently available for this article.
Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative